In Vitro Diagnostics (IVD) Market to Worth USD 126.6 Billion by 2032, Growing at 4.8% CAGR – SNS Insider
Technological innovations and aging populations are major factors fueling the growth of the IVD market
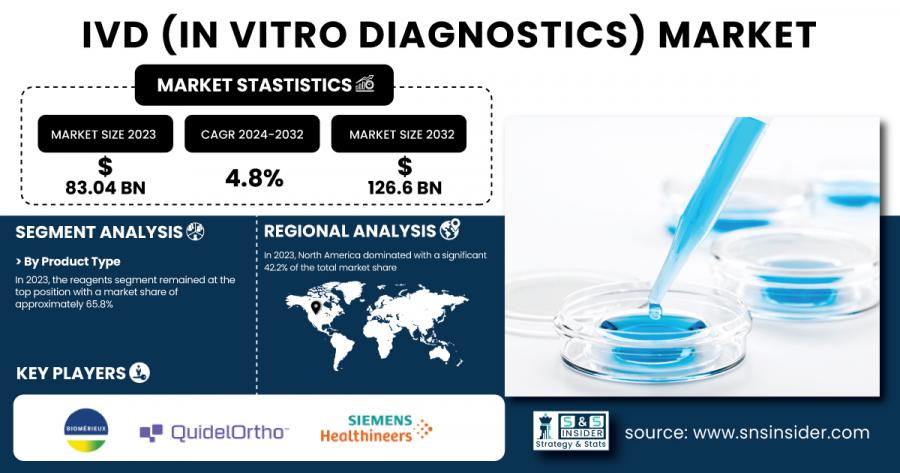
AUSTIN, TX, UNITED STATES, February 12, 2025 /EINPresswire.com/ -- According to Research by SNS Insider, The IVD (In Vitro Diagnostics) Market size was estimated at USD 83.04 billion in 2023 and is expected to reach USD 126.6 billion by 2032 at a CAGR of 4.8% during the forecast period of 2024-2032.
The IVD (In Vitro Diagnostics) market is rapidly expanding with increased prevalence of noncommunicable diseases and rising demand for accurate and fast diagnostics. There has been improvement in diagnostic precision and speed through technological advancements such as automated systems and innovations, like AAV5 DetectCDx and APOA2-iTQ. Rising awareness, an aging population, and financial pressures in the healthcare sector make the market poised to grow substantially.
Get a Free Sample Report@ https://www.snsinsider.com/sample-request/4700
By Product Type: The instruments segment is the second largest in 2023 and is expected to grow at the fastest pace.
Because of the dominance of reagents and consumables, Intruments constitute a larger share of the market. Instruments are very important for the performance of diagnostic tests but need to be regularly maintained, replaced, and calibrated, making them less frequently purchased than consumables such as reagents, antibodies, and test kits. Additionally, instruments being more expensive and having a longer life, their turnover is limited and not frequently replaced. Hence, consumables that have to be replenished frequently, add to the revenue stream in the IVD market.
The reagents segment dominated the market with 65.8% of market share in 2023, mainly as there has been consistent R&D to introduce newer reagents and kits for various test kits like Onclarity HPV Assay BD, thereby more emphasis towards precision medicine boosting demand for the innovation of the new reagent and, through such continuous upgradations for precise diagnostics, growth is foreseen for this reagents market due to rising demands for quick diagnostics tools and tests.
By Technology: The immunoassay segment dominated the IVD market in 2023.
Driven by the rising prevalence of chronic and communicable diseases, which highlights the need for early diagnosis. Immunological methods, especially Enzyme-Linked Immunosorbent Assays (ELISAs), are increasingly in demand for their effectiveness in early disease detection. Major industry players, such as Sysmex Corporation and Fujirebio Holdings, are heavily investing in R&D to innovate diagnostic tools in the immunoassay field, thereby improving diagnostic accuracy and expanding their market share. The coagulation segment projected the fastest growth with the highest CAGR between 2024 and 2032, driven by the increasing cases of cardiovascular diseases, blood disorders, and autoimmune conditions.
By Test Location: The homecare segment projected the fastest growth at a high compound annual growth rate from 2024 to 2032.
Factors such as the rising reliability of in-home tests and the adoption of the patient-centric approach from manufacturers will enhance the segment. These tests greatly helped control the spread of the SARS-CoV-2 pandemic. Global governments have been taking extreme steps to provide cheap and effective testing through home care. For example, in April 2023, Llusern Scientific, a spin-out from the University of South Wales, developed the Lodestar Dx platform, which is a PoC molecular diagnostic tool for panel testing of urinary tract infections.
By Application: The oncology segment is anticipated to grow at the fastest rate from 2024 to 2032.
Due to the rising incidence and high mortality rates of cancer, thereby driving the demand for early-stage cancer biomarker tests. Besides, the growth is further spurred by the growing approval of novel tests, continuous research and development activities, and favorable initiatives by the regulatory bodies. For instance, in June 2023, the U.S. FDA launched a pilot program to enable manufacturers to submit validation and performance data for cancer-related LDTs. Moreover, in April 2023, Biocartis Group NV and APIS Assay Technologies Ltd. collaborated to develop and commercialize a breast cancer subtyping test on the Idylla molecular diagnostics platform.
Need any customization research on IVD (In Vitro Diagnostics) Market, Enquire Now@ https://www.snsinsider.com/enquiry/4700
By End-user: The hospital segment dominated the market and accounted for the highest market share in the IVD market in 2023.
The dominance is driven by the increase in hospitalizations that require fast diagnostic support. The growth in healthcare infrastructure, along with initiatives from the government, is further expected to improve the facilities offered by hospitals and thus increase demand for hospital-based IVD tests. Hospitals also purchase and consume a large quantity of IVD devices and therefore play a vital role in important decision-making processes. More than 6,129 hospitals in the U.S. relied on IVD in 2023 to provide patients with timely and accurate test results.
Regional Analysis: In 2023, North America dominated the market with a 42.2% market share
The dominance is due to the growing number of chronic diseases, the government's solid support, and new test releases, such as the BD and CerTest Biotec PCR test for Mpox. These factors are anticipated to be sustained because of the need for genetic testing and personalized health care, primarily for diabetes and cancer. The Asia Pacific region is going to experience fastest growth from 2024 to 2032, triggered by economic improvements, urbanization, and collaborations like those within Fapon's collaboration with Halodoc in Indonesia, increasing in vitro diagnostic services.
Key Players in IVD (In Vitro Diagnostics) Market
• Abbott
• bioMérieux SA
• QuidelOrtho Corporation
• Siemens Healthineers AG
• Bio-Rad Laboratories Inc.
• Qiagen
• Sysmex Corporation
• Charles River Laboratories
• Quest Diagnostics Incorporated
• Agilent Technologies Inc.
• Danaher Corporation
• BD
• F. Hoffmann-La Roche Ltd.
• Seegene Inc.
• DiaSorin S.p.A.
Buy Full Research Report on IVD (In Vitro Diagnostics) Market 2024-2032 @ https://www.snsinsider.com/checkout/4700
Table of Contents – Major Key Points
1. Introduction
2. Executive Summary
3. Research Methodology
4. Market Dynamics Impact Analysis
5. Statistical Insights and Trends Reporting
6. Competitive Landscape
7. IVD (In Vitro Diagnostics) Market by Product Type
8. IVD (In Vitro Diagnostics) Market by Technology
9. IVD (In Vitro Diagnostics) Market by Test Location
10. IVD (In Vitro Diagnostics) Market by Application
11. IVD (In Vitro Diagnostics) Market by End-user
12. Regional Analysis
13. Company Profiles
14. Use Cases and Best Practices
15. Conclusion
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Jagney Dave
SNS Insider Pvt. Ltd
+1 315-636-4242
email us here
Visit us on social media:
Facebook
X
LinkedIn
Instagram
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release